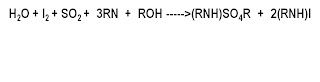Thursday 26 July 2012
Water determination by Karlfischer reagent(KF)
Water determination
Reference USP General Chapter<921>
Simple Reaction :
water against KF Reagent(Karlfischer reagent)
ROH The solvent is generally methanol.
Methanol is the common solvent used as media
When analysing Aldehydes and ketones, do not use methanol as a media. these compounds reacts with methanol to form additional water.
No. of iodines is equivalent to no. water molecules in the rection of iodine consumtion.
KF degrades itself with atmospheric air and moisture, since the oxidation happening to sulfer dioxide.
so that the standardisation of KF should be done frequently(Daily once).
Each ml of KF can neutralise(here react to cosume) 5-6 mg of water. this will be exactly known by standardisation of KF with DST(Disodium tartarate dihydrate) or Water.
Commercially kf reagents avialable in two types with respect to concentration.
1) 2 mg/ ml
2) 5 mg/ml
Calibration of KF Apparatus as per USP<921>
Standardisation of KF Reagent :
Weigh the DST( previously dried for 3 hours at 105 degrees) about 0.2 g and titrate with KF.
Formula: weight of the sample X 0.1566 X 1000
Titration volume(KF Consumed)
This 0.1566 comes from ratio of molecular wt of water divided by DST molecular wt.
This standardisation can be done with water(Take pure water only)
Formula : wt of the sample x 1000
Titration volume(KF Consumed)
Here 0.1566 is not involved, since we are standardisint with water. so water molecular wt divided by water molecular wt. gives 1.
Note :
Take the sample to consume at least 10-30% of the burette volume so as to get accurate and reproducible results.
Water content formula : TV x KF Factor x 100
Wt.of the sample(g) x 1000
Second advanced method for lower levels of Water determination in the range of 5 ppm to 5.0%.
This method is called water content by Coloumetry.
Here there is no Burette. The KF Reagent it self act as a generator of iodine in the vessel containing two platinum electrodes.
The reagent(KF for Coulometry) contains iodine in the form of Iodine Ion.
This iodine Ion oxidise to get free iodine which reacts with water.
No need to change the reagent for each sample.
Reference USP General Chapter<921>
Simple Reaction :
water against KF Reagent(Karlfischer reagent)
ROH The solvent is generally methanol.
Methanol is the common solvent used as media
When analysing Aldehydes and ketones, do not use methanol as a media. these compounds reacts with methanol to form additional water.
No. of iodines is equivalent to no. water molecules in the rection of iodine consumtion.
KF degrades itself with atmospheric air and moisture, since the oxidation happening to sulfer dioxide.
so that the standardisation of KF should be done frequently(Daily once).
Each ml of KF can neutralise(here react to cosume) 5-6 mg of water. this will be exactly known by standardisation of KF with DST(Disodium tartarate dihydrate) or Water.
Commercially kf reagents avialable in two types with respect to concentration.
1) 2 mg/ ml
2) 5 mg/ml
Calibration of KF Apparatus as per USP<921>
| <921> 1)water or DST can be used for KF standardisation, 2) while testing, the KF Consumtion should be at least 30% of burette volume, this condition is for the purpose of accurate result, 3) Precision & accuracy of the water standard or DST can be verified |
Standardisation of KF Reagent :
Weigh the DST( previously dried for 3 hours at 105 degrees) about 0.2 g and titrate with KF.
Formula: weight of the sample X 0.1566 X 1000
Titration volume(KF Consumed)
This 0.1566 comes from ratio of molecular wt of water divided by DST molecular wt.
This standardisation can be done with water(Take pure water only)
Formula : wt of the sample x 1000
Titration volume(KF Consumed)
Here 0.1566 is not involved, since we are standardisint with water. so water molecular wt divided by water molecular wt. gives 1.
Note :
Take the sample to consume at least 10-30% of the burette volume so as to get accurate and reproducible results.
Water content formula : TV x KF Factor x 100
Wt.of the sample(g) x 1000
Second advanced method for lower levels of Water determination in the range of 5 ppm to 5.0%.
This method is called water content by Coloumetry.
Here there is no Burette. The KF Reagent it self act as a generator of iodine in the vessel containing two platinum electrodes.
The reagent(KF for Coulometry) contains iodine in the form of Iodine Ion.
This iodine Ion oxidise to get free iodine which reacts with water.
The solvent (generally methanol) is involved in the reaction
A suitable base keeps the pH 5 - 7
Thursday 19 July 2012
pH meter and good practices in pH
pH is -log[H+]
we use pH meter to check the pH of the sample solution.
pH meter does not know about pH.
the pH electode conducts charge from sample in milli volts(mV)
This mV gets converted into pH units
(0-14 is the scale of pH)
1 pH unit
Approximately 59 millivolts per pH unit
normally if pH is 7, it is neutral, no coduction of charge from the sample solution , so that the net charge mV=0.
at pH=8, the mV=59
at pH=9, the mV=59 x 2=118
at pH=10, tge mV=59 x 3= 177 like wise pH meter works for 7-14 pH.
at pH=6 the value will be negative
that is -59.
at pH=5 the mV= -118.
at pH=4 the mV= -177 like wise pH meter works for 7-0 pH.
AlwayspH is electrode keep in a buffer solution or in tap water or 0.1 M HCl when not in use.
Do not keep in Purified water(DM or DI Water),
Reason: the electode sensitivity will be damaged, if it is kept is purified water.
Calibration .USP<791>
Select two buffers such that the sample pH should fall between the range.
pH meter accuracy with buffer as a standard +/- 0.04
Analysis:
Always verify the pH meter with one standard buffer when analysing the pH of a sample .
Take the stable readings only..
we use pH meter to check the pH of the sample solution.
pH meter does not know about pH.
the pH electode conducts charge from sample in milli volts(mV)
This mV gets converted into pH units
(0-14 is the scale of pH)
1 pH unit
Approximately 59 millivolts per pH unit
normally if pH is 7, it is neutral, no coduction of charge from the sample solution , so that the net charge mV=0.
at pH=8, the mV=59
at pH=9, the mV=59 x 2=118
at pH=10, tge mV=59 x 3= 177 like wise pH meter works for 7-14 pH.
at pH=6 the value will be negative
that is -59.
at pH=5 the mV= -118.
at pH=4 the mV= -177 like wise pH meter works for 7-0 pH.
AlwayspH is electrode keep in a buffer solution or in tap water or 0.1 M HCl when not in use.
Do not keep in Purified water(DM or DI Water),
Reason: the electode sensitivity will be damaged, if it is kept is purified water.
Calibration .USP<791>
Select two buffers such that the sample pH should fall between the range.
pH meter accuracy with buffer as a standard +/- 0.04
Analysis:
Always verify the pH meter with one standard buffer when analysing the pH of a sample .
Take the stable readings only..
Wednesday 18 July 2012
Photo stability
Photo stability :
We check the stability of samples under environmental condition such as light(photo).
An artificial light similar to natural light will be exposed to the samples and check the quality before expore and after exposure will be monitored and justified.
The intrinsic photostability characteristics of new drug substances and products should be
evaluated to demonstrate that, as appropriate, light exposure does not result in
unacceptable change. Normally, photostability testing is carried out on a single batch
It happens in two ways.
1. Confirmatory studies
For confirmatory studies, samples should be exposed to light providing an overall
illumination of not less than 1.2 million lux hours and an integrated near ultraviolet energy of not less than 200 watt hours/square meter to allow direct comparisons to be made between the drug substance and drug product.
2.Forced degradation(stress testing)
To provide the analytical method is stability indicating, the exposure will be very high than confirmaroty testing.
Sample analysis :
At the end of the exposure period, the samples should be examined for any changes in Quality.
Step :1 expose directly and analyse the sample, if sample passes its specification, no futher testing, study is completed.
If fails at step 1, expose on primary packing,if sample passes its specification, no futher testing, study is completed.
If fails at step 2, expose on sencondar packing,
If fails, change the packing style and study it once again.
Procedure for Light energy verification :Spread about 5 g of sample in a transparent Petri dish and expose to light for three times cycle to 1200 KLUX hours and 200 Watt-Hours / Sq.mts.Spread about 5 g of sample in a transparent Petri dish and expose to light for three times cycle to 1200 KLUX hours and 200 Watt-Hours / Sq.mts.
We check the stability of samples under environmental condition such as light(photo).
An artificial light similar to natural light will be exposed to the samples and check the quality before expore and after exposure will be monitored and justified.
The intrinsic photostability characteristics of new drug substances and products should be
evaluated to demonstrate that, as appropriate, light exposure does not result in
unacceptable change. Normally, photostability testing is carried out on a single batch
It happens in two ways.
1. Confirmatory studies
For confirmatory studies, samples should be exposed to light providing an overall
illumination of not less than 1.2 million lux hours and an integrated near ultraviolet energy of not less than 200 watt hours/square meter to allow direct comparisons to be made between the drug substance and drug product.
2.Forced degradation(stress testing)
To provide the analytical method is stability indicating, the exposure will be very high than confirmaroty testing.
Sample analysis :
At the end of the exposure period, the samples should be examined for any changes in Quality.
Step :1 expose directly and analyse the sample, if sample passes its specification, no futher testing, study is completed.
If fails at step 1, expose on primary packing,if sample passes its specification, no futher testing, study is completed.
If fails at step 2, expose on sencondar packing,
If fails, change the packing style and study it once again.
Procedure for Light energy verification :Spread about 5 g of sample in a transparent Petri dish and expose to light for three times cycle to 1200 KLUX hours and 200 Watt-Hours / Sq.mts.Spread about 5 g of sample in a transparent Petri dish and expose to light for three times cycle to 1200 KLUX hours and 200 Watt-Hours / Sq.mts.
Note: If the degradation is more than 20% for 1200 KLUX hours and 200 Watt-Hours /Sq.mts three times cycle sample, perform the analysis on 1200 KLUX hours and 200 Watt-Hours / Sq.mts two times cycle sample.Establish the Actinometric system simultaneously for the corresponding sample, employing the procedure ICH Q1B Option –1.
Actinometric standard solution: Use 2% W/V Qunine monohydrochloride DihydrateAqueous solution. Option –1: Put 10 milliliters (mL) of the solution into a 20 mL colorless ampoule seal it hermetically(air tight), and use this as the sample. Separately, put 10 mL of the solution into a 20 ml
colourless ampoule, seal it hermitically, wrap in aluminum foil to protect completely from light, and use this as the control. Expose the sample and control to the light source for an appropriate number of hours. After exposure determine the absorbances of the sample (AT) and the control (Ao) at 400 nm using a 1 centimeter (cm) path length. Calculate the change inabsorbance,
∆ A = AT – AO.
Acceptance criteria: The length of exposure should be sufficient to ensure a change in absorbance of at least 0.9.
Stability chambers
Stability Chambers :
Whenever Stability Chambers are not maintained to its controlled conditions
The reasons may be
First check the INPUTS and OUT PUTS in software ICDAS 1.2 and act against the particular error, Few of the experiences
A. When software showing mains off
1. Ensure the power switched on
2. Ensure the mains are not tripped down
Due to the above two reasons both the temperature and humidity will fall down
B. When software showing Boiler heaters off
1. Ensure the water level in the reservoir tank
2. Then check WL1,WL2 in the chamber screen, when any of WL1,WL2 is not displaying on the screen, replace with new float switch.
3. Check the boiler heater function with the help of electrician.
C. When software showing No error, but not maintaining
1. Ensure the circulation motor on back side of the chamber is rotating or not
2. Check the relay of the chamber.
Chamber events
1. Daily chamber log
2. Cleaning record-Quarterly
3. Chamber history card entry for every failure of event.
4. Deviation – More than 3 consecutive events fail.
5.replace the samples, if a chamber failed to maintains more than 24 hours.(As per ICH)
Subscribe to:
Posts (Atom)
Stability of drug substances
Stability studies : Define stability : To study on how the product quality changes with time under the influence of temperature, humi...
-
Water determination Reference USP General Chapter<921> Simple Reaction : water against KF Reagent(Karlfischer reagent) ROH...
-
Ho w much duration of time to hold the samples in a Photostability chamber. ICH Defines, Expose only one batch is enough to 1.2 mill...
-
Dirty equipment Hold time studies : Now a days the auditors are asking about the Dirty equipment Hold time study report in cleaning va...