Water determination
Reference USP General Chapter<921>
Simple Reaction :
water against KF Reagent(Karlfischer reagent)
ROH The solvent is generally methanol.
Methanol is the common solvent used as media
When analysing Aldehydes and ketones, do not use methanol as a media. these compounds reacts with methanol to form additional water.
No. of iodines is equivalent to no. water molecules in the rection of iodine consumtion.
KF degrades itself with atmospheric air and moisture, since the oxidation happening to sulfer dioxide.
so that the standardisation of KF should be done frequently(Daily once).
Each ml of KF can neutralise(here react to cosume) 5-6 mg of water. this will be exactly known by standardisation of KF with DST(Disodium tartarate dihydrate) or Water.
Commercially kf reagents avialable in two types with respect to concentration.
1) 2 mg/ ml
2) 5 mg/ml
Calibration of KF Apparatus as per USP<921>
Standardisation of KF Reagent :
Weigh the DST( previously dried for 3 hours at 105 degrees) about 0.2 g and titrate with KF.
Formula: weight of the sample X 0.1566 X 1000
Titration volume(KF Consumed)
This 0.1566 comes from ratio of molecular wt of water divided by DST molecular wt.
This standardisation can be done with water(Take pure water only)
Formula : wt of the sample x 1000
Titration volume(KF Consumed)
Here 0.1566 is not involved, since we are standardisint with water. so water molecular wt divided by water molecular wt. gives 1.
Note :
Take the sample to consume at least 10-30% of the burette volume so as to get accurate and reproducible results.
Water content formula : TV x KF Factor x 100
Wt.of the sample(g) x 1000
Second advanced method for lower levels of Water determination in the range of 5 ppm to 5.0%.
This method is called water content by Coloumetry.
Here there is no Burette. The KF Reagent it self act as a generator of iodine in the vessel containing two platinum electrodes.
The reagent(KF for Coulometry) contains iodine in the form of Iodine Ion.
This iodine Ion oxidise to get free iodine which reacts with water.
No need to change the reagent for each sample.
Reference USP General Chapter<921>
Simple Reaction :
water against KF Reagent(Karlfischer reagent)
ROH The solvent is generally methanol.
Methanol is the common solvent used as media
When analysing Aldehydes and ketones, do not use methanol as a media. these compounds reacts with methanol to form additional water.
No. of iodines is equivalent to no. water molecules in the rection of iodine consumtion.
KF degrades itself with atmospheric air and moisture, since the oxidation happening to sulfer dioxide.
so that the standardisation of KF should be done frequently(Daily once).
Each ml of KF can neutralise(here react to cosume) 5-6 mg of water. this will be exactly known by standardisation of KF with DST(Disodium tartarate dihydrate) or Water.
Commercially kf reagents avialable in two types with respect to concentration.
1) 2 mg/ ml
2) 5 mg/ml
Calibration of KF Apparatus as per USP<921>
| <921> 1)water or DST can be used for KF standardisation, 2) while testing, the KF Consumtion should be at least 30% of burette volume, this condition is for the purpose of accurate result, 3) Precision & accuracy of the water standard or DST can be verified |
Standardisation of KF Reagent :
Weigh the DST( previously dried for 3 hours at 105 degrees) about 0.2 g and titrate with KF.
Formula: weight of the sample X 0.1566 X 1000
Titration volume(KF Consumed)
This 0.1566 comes from ratio of molecular wt of water divided by DST molecular wt.
This standardisation can be done with water(Take pure water only)
Formula : wt of the sample x 1000
Titration volume(KF Consumed)
Here 0.1566 is not involved, since we are standardisint with water. so water molecular wt divided by water molecular wt. gives 1.
Note :
Take the sample to consume at least 10-30% of the burette volume so as to get accurate and reproducible results.
Water content formula : TV x KF Factor x 100
Wt.of the sample(g) x 1000
Second advanced method for lower levels of Water determination in the range of 5 ppm to 5.0%.
This method is called water content by Coloumetry.
Here there is no Burette. The KF Reagent it self act as a generator of iodine in the vessel containing two platinum electrodes.
The reagent(KF for Coulometry) contains iodine in the form of Iodine Ion.
This iodine Ion oxidise to get free iodine which reacts with water.
The solvent (generally methanol) is involved in the reaction
A suitable base keeps the pH 5 - 7
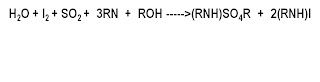





what was the acceptance criteria between two determination of kf standardization and why?
ReplyDeleteWhat is Water factor limits?
ReplyDelete5to 6
DeleteFactors limit : 4.50- 5.50
DeletePretty nice post. I just stumbled upon your weblog and wanted to say that I have really enjoyed browsing your blog posts. After all I’ll be subscribing to your feed and I hope you write again soon! Lorazepam
ReplyDeleteI'm glad to see the great detail here!. custom embroidered key tags
ReplyDeleteThey found and fixed hidden damage too. Crown Mold Specialists
ReplyDeleteFast, efficient, and trustworthy service. Repipe in houston
ReplyDelete